Hi there,
I am trying to develop a method that starts with samples that have been centrifuged and contain a pellet at the bottom on a 15 mL tube.
I want to aspirate and discard just the supernatant and leave the pellet.
Does anyone have any advice on the best way to do this ?
I noticed ‘aspirate 2nd phase’ . Does anyone have experience with this ? What would be the best settings for aspirate position ? Do the tips some how detect the second phase or should I just try to optimize a volume to aspirate?
Thanks
@awaller - Please review the thread linked below, which goes into some detail about how this can be done using TADM. There are numerous links within the thread that contain more background information and context.
Detecting clogs using TADM
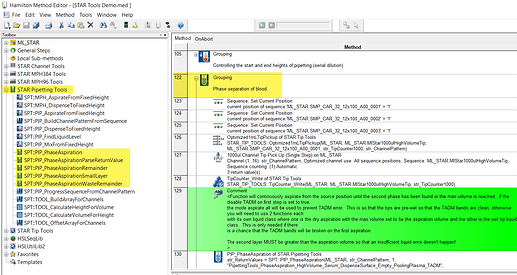
There are also resources in the STAR tools suite, within the pipetting tools library of the suite. I would review the Phase Separation grouping within the demo method that imports with the library to get an idea of the implementation, but you will want to customize for a simpler protocol. STAR tools can be found in Eric’s materials upload link.
-Nick
Thanks.
I’ll have a look at the TADM material.
I"m using a Vantage, do the STAR tools also work with that ?
thanks
Nick’s recommendation provides the most flexibility and options. The default Aspirate Second Phase command does allow for the detection of the interface between the top phase and the bottom phase of bi-layer liquid and is ideal when using organic solvents and only the bottom layer or top layer must be aspirated. However, it requires the use of Slim Tips to prevent overflow and is not customizable.
I’m trying to use the STAR tools PIP Phase_Aspiration . Some of the liquid classes with TADM info were defined for non-filter tips. If we are using filter tips will be have to redefine the TADM curves or can we just set change the tip type in the definition ?
Thanks
This is difficult to answer without seeing the sets of curves side by side or superimposed on the same plot (filtered vs non-filtered at the volumes of interest). It also depends on how sensitive you want your detection of phase change, given any potential differences in pressure curve characteristics between the two tip types.
For TADM-based applications of this nature, I have always developed bands based off of pressure curves of a consistent tip type. Additionally, I like to keep tip definitions on deck layout true to the physical rack, so barcode masks are accurate and liquid classes match the intended tip type.
That being said, you can always pull up two sets of curves in the liquid editor and make a judgement call. Just perform a transfer set using filtered and non-filtered and see how they line up.
-Nick
1 Like
If you are aiming to get proof of function or test code, then yes you can update the tip type to match the liquid class used for the aspiration/dispense. For optimization, you will certainly want to tailor the TADM gaurdbands for the tips that will be used.
-Nick
1 Like
Given the fact that your pellet is approximately at the same position:
How about empirically estimating the best fix height for getting the most liquid into the tip and at the same time having a minimal dead volume in/on the pellet which does not affect your downstream experiments?
Also, lowering flow rate and adjusting other aspiration parameters could be an option.
We have something similar, however, we use only the supernatant and discard the pellet. So we adjusted our experiment in order to have enough volume above the pellet and then aspirate from a fixed height.
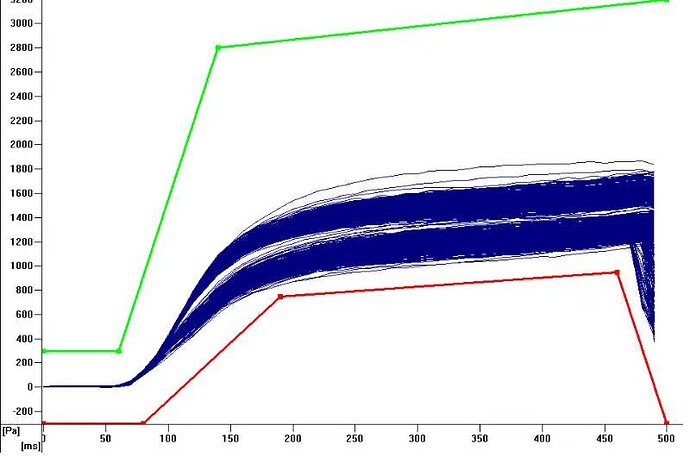
filter is a barrier that will slow air going into tip, and effect pressure. So curve for tip with and without filer will have a shift as following image, which may lead fake clot error. So use the right tip or redefine tadm curve